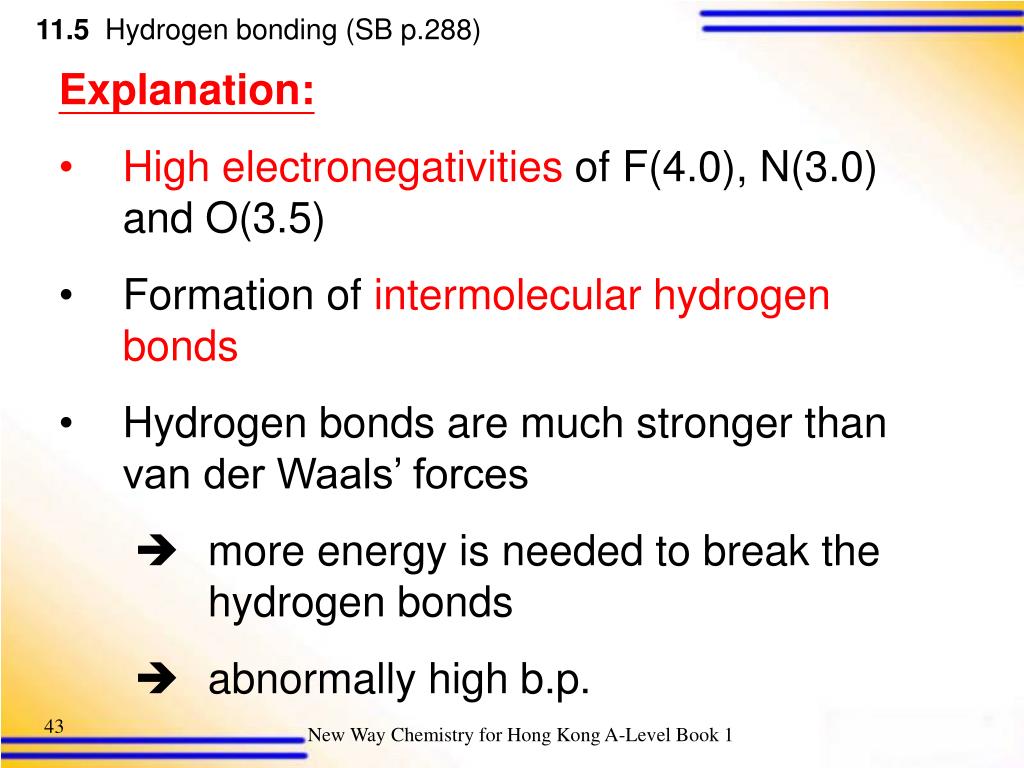
Strength Of H Bond In H2O And Hf . thus hydrogen bonding can account for the unusually high boiling points of nh 3, h 2 o, and hf. Hydrogen bonds are stronger than van der waals forces. in the cases of nh 3, h 2 o and hf there must be some additional intermolecular forces of attraction, requiring significantly more heat energy to break. Describe the structure, such as it is,. h−f h − f bond is stronger in comparison to o−h o − h bond. explain what is meant by hydrogen bonding and the molecular structural features that bring it about. In the case of h−f h − f, there exists hydrogen bonding even in vapour. i know that acidity of an acid increases across a period (with electronegativity increase of the atom. hydrogen bonds have about a tenth of the strength of an average covalent bond, and are constantly broken and.
from www.slideserve.com
in the cases of nh 3, h 2 o and hf there must be some additional intermolecular forces of attraction, requiring significantly more heat energy to break. hydrogen bonds have about a tenth of the strength of an average covalent bond, and are constantly broken and. h−f h − f bond is stronger in comparison to o−h o − h bond. thus hydrogen bonding can account for the unusually high boiling points of nh 3, h 2 o, and hf. Describe the structure, such as it is,. explain what is meant by hydrogen bonding and the molecular structural features that bring it about. In the case of h−f h − f, there exists hydrogen bonding even in vapour. Hydrogen bonds are stronger than van der waals forces. i know that acidity of an acid increases across a period (with electronegativity increase of the atom.
PPT Intermolecular Forces PowerPoint Presentation, free download ID
Strength Of H Bond In H2O And Hf thus hydrogen bonding can account for the unusually high boiling points of nh 3, h 2 o, and hf. thus hydrogen bonding can account for the unusually high boiling points of nh 3, h 2 o, and hf. explain what is meant by hydrogen bonding and the molecular structural features that bring it about. Describe the structure, such as it is,. In the case of h−f h − f, there exists hydrogen bonding even in vapour. hydrogen bonds have about a tenth of the strength of an average covalent bond, and are constantly broken and. i know that acidity of an acid increases across a period (with electronegativity increase of the atom. h−f h − f bond is stronger in comparison to o−h o − h bond. Hydrogen bonds are stronger than van der waals forces. in the cases of nh 3, h 2 o and hf there must be some additional intermolecular forces of attraction, requiring significantly more heat energy to break.
From www.youtube.com
MDCAT53 Strength of hydrogen bond Strength of H bonding Strength Of H Bond In H2O And Hf thus hydrogen bonding can account for the unusually high boiling points of nh 3, h 2 o, and hf. explain what is meant by hydrogen bonding and the molecular structural features that bring it about. In the case of h−f h − f, there exists hydrogen bonding even in vapour. Describe the structure, such as it is,. . Strength Of H Bond In H2O And Hf.
From www.slideshare.net
Hydrogen Bonding Powerpoint Strength Of H Bond In H2O And Hf explain what is meant by hydrogen bonding and the molecular structural features that bring it about. Hydrogen bonds are stronger than van der waals forces. thus hydrogen bonding can account for the unusually high boiling points of nh 3, h 2 o, and hf. i know that acidity of an acid increases across a period (with electronegativity. Strength Of H Bond In H2O And Hf.
From www.researchgate.net
The hydrogen bond distances in D associates Download Table Strength Of H Bond In H2O And Hf In the case of h−f h − f, there exists hydrogen bonding even in vapour. h−f h − f bond is stronger in comparison to o−h o − h bond. Describe the structure, such as it is,. i know that acidity of an acid increases across a period (with electronegativity increase of the atom. in the cases. Strength Of H Bond In H2O And Hf.
From www.slideserve.com
PPT Chapter 11 PowerPoint Presentation, free download ID5852920 Strength Of H Bond In H2O And Hf explain what is meant by hydrogen bonding and the molecular structural features that bring it about. hydrogen bonds have about a tenth of the strength of an average covalent bond, and are constantly broken and. Hydrogen bonds are stronger than van der waals forces. i know that acidity of an acid increases across a period (with electronegativity. Strength Of H Bond In H2O And Hf.
From byjus.com
the order of basic strength of CH4 HF NH3 H2O Strength Of H Bond In H2O And Hf Describe the structure, such as it is,. thus hydrogen bonding can account for the unusually high boiling points of nh 3, h 2 o, and hf. hydrogen bonds have about a tenth of the strength of an average covalent bond, and are constantly broken and. i know that acidity of an acid increases across a period (with. Strength Of H Bond In H2O And Hf.
From ericson1010ufkstudyquizz.z14.web.core.windows.net
Intermolecular Forces Examples With Answers Strength Of H Bond In H2O And Hf thus hydrogen bonding can account for the unusually high boiling points of nh 3, h 2 o, and hf. Describe the structure, such as it is,. i know that acidity of an acid increases across a period (with electronegativity increase of the atom. h−f h − f bond is stronger in comparison to o−h o − h. Strength Of H Bond In H2O And Hf.
From www.toppr.com
7. 9 120 (D) CH Which of the following has the strongest hydrogen Strength Of H Bond In H2O And Hf In the case of h−f h − f, there exists hydrogen bonding even in vapour. thus hydrogen bonding can account for the unusually high boiling points of nh 3, h 2 o, and hf. i know that acidity of an acid increases across a period (with electronegativity increase of the atom. Describe the structure, such as it is,.. Strength Of H Bond In H2O And Hf.
From byjus.com
30.Order of strength of melting point HF,H2O,NH3 Strength Of H Bond In H2O And Hf i know that acidity of an acid increases across a period (with electronegativity increase of the atom. In the case of h−f h − f, there exists hydrogen bonding even in vapour. hydrogen bonds have about a tenth of the strength of an average covalent bond, and are constantly broken and. Describe the structure, such as it is,.. Strength Of H Bond In H2O And Hf.
From questions-in.kunduz.com
4. Hydrogen bonds are formed in many com... Chemistry Strength Of H Bond In H2O And Hf i know that acidity of an acid increases across a period (with electronegativity increase of the atom. Hydrogen bonds are stronger than van der waals forces. h−f h − f bond is stronger in comparison to o−h o − h bond. in the cases of nh 3, h 2 o and hf there must be some additional. Strength Of H Bond In H2O And Hf.
From www.pathwaystochemistry.com
Table of Bond Energies Pathways to Chemistry Strength Of H Bond In H2O And Hf in the cases of nh 3, h 2 o and hf there must be some additional intermolecular forces of attraction, requiring significantly more heat energy to break. Describe the structure, such as it is,. hydrogen bonds have about a tenth of the strength of an average covalent bond, and are constantly broken and. h−f h − f. Strength Of H Bond In H2O And Hf.
From www.semanticscholar.org
Hydrogen Bonding Semantic Scholar Strength Of H Bond In H2O And Hf Describe the structure, such as it is,. In the case of h−f h − f, there exists hydrogen bonding even in vapour. in the cases of nh 3, h 2 o and hf there must be some additional intermolecular forces of attraction, requiring significantly more heat energy to break. explain what is meant by hydrogen bonding and the. Strength Of H Bond In H2O And Hf.
From www.youtube.com
How do Hydrogen bonds form in H2O NH3 HF Hydrogen Bonding Strength Of H Bond In H2O And Hf thus hydrogen bonding can account for the unusually high boiling points of nh 3, h 2 o, and hf. i know that acidity of an acid increases across a period (with electronegativity increase of the atom. Hydrogen bonds are stronger than van der waals forces. In the case of h−f h − f, there exists hydrogen bonding even. Strength Of H Bond In H2O And Hf.
From www.slideshare.net
Intermolecular forces Strength Of H Bond In H2O And Hf In the case of h−f h − f, there exists hydrogen bonding even in vapour. in the cases of nh 3, h 2 o and hf there must be some additional intermolecular forces of attraction, requiring significantly more heat energy to break. explain what is meant by hydrogen bonding and the molecular structural features that bring it about.. Strength Of H Bond In H2O And Hf.
From brilliant.org
Hydrogen Bonds Brilliant Math & Science Wiki Strength Of H Bond In H2O And Hf i know that acidity of an acid increases across a period (with electronegativity increase of the atom. thus hydrogen bonding can account for the unusually high boiling points of nh 3, h 2 o, and hf. hydrogen bonds have about a tenth of the strength of an average covalent bond, and are constantly broken and. h−f. Strength Of H Bond In H2O And Hf.
From www.toppr.com
Hydrogen bonds are formed in many compounds e.g., H2O, HF and NH3 . The Strength Of H Bond In H2O And Hf h−f h − f bond is stronger in comparison to o−h o − h bond. explain what is meant by hydrogen bonding and the molecular structural features that bring it about. in the cases of nh 3, h 2 o and hf there must be some additional intermolecular forces of attraction, requiring significantly more heat energy to. Strength Of H Bond In H2O And Hf.
From byjus.com
the hydrogen bonds encountered in HF,H_2O,NH_3 and HF_2^ .The relative Strength Of H Bond In H2O And Hf h−f h − f bond is stronger in comparison to o−h o − h bond. i know that acidity of an acid increases across a period (with electronegativity increase of the atom. In the case of h−f h − f, there exists hydrogen bonding even in vapour. explain what is meant by hydrogen bonding and the molecular. Strength Of H Bond In H2O And Hf.
From chemistryskills.com
Hydrogen Bonding Chemistry Skills Strength Of H Bond In H2O And Hf thus hydrogen bonding can account for the unusually high boiling points of nh 3, h 2 o, and hf. hydrogen bonds have about a tenth of the strength of an average covalent bond, and are constantly broken and. Hydrogen bonds are stronger than van der waals forces. h−f h − f bond is stronger in comparison to. Strength Of H Bond In H2O And Hf.
From www3.nd.edu
Hydrogen Bond Lengths in Biomolecules Strength Of H Bond In H2O And Hf thus hydrogen bonding can account for the unusually high boiling points of nh 3, h 2 o, and hf. In the case of h−f h − f, there exists hydrogen bonding even in vapour. Describe the structure, such as it is,. hydrogen bonds have about a tenth of the strength of an average covalent bond, and are constantly. Strength Of H Bond In H2O And Hf.